Note: There’s a summary table at the end of this page.
Definitions
Organic: Contains carbon
Hydrocarbons: Organic compounds made from hydrogen and carbon only
Alkanes: Organic compounds that come from crude oil
Homologous series: A series of compounds with the same functional group and similar chemical properties
Saturated: Compounds that don’t have a carbon carbon (c=c) double bond (like alkanes)
General formula
General formula for alkanes: CnH2n+n
Each homologous series has its own general formula.
To use the general formula, replace n in the general formula with the number of C (carbon) atoms.
eg. An alkane compound with 3 carbon atoms:
CnH2n+2
= C(3)H2(3)+2
= C3H8
How to name alkanes
Every alkane ends with “-ane”, but starts differently depending on the number of C (carbon) atoms.
1 – meth
2 – eth
3 – prop
4 – but
5 – pent
6 – hex
eg. An alkane compound with 4 carbon atoms: butane
Here’s a trick I use to remember how each compounds starts:
(1) Monkeys (2) Eat (3) Peanut (4) Butter
5 & 6 are easy enough to remember because they are the same as shapes (pentagon, hexagon)
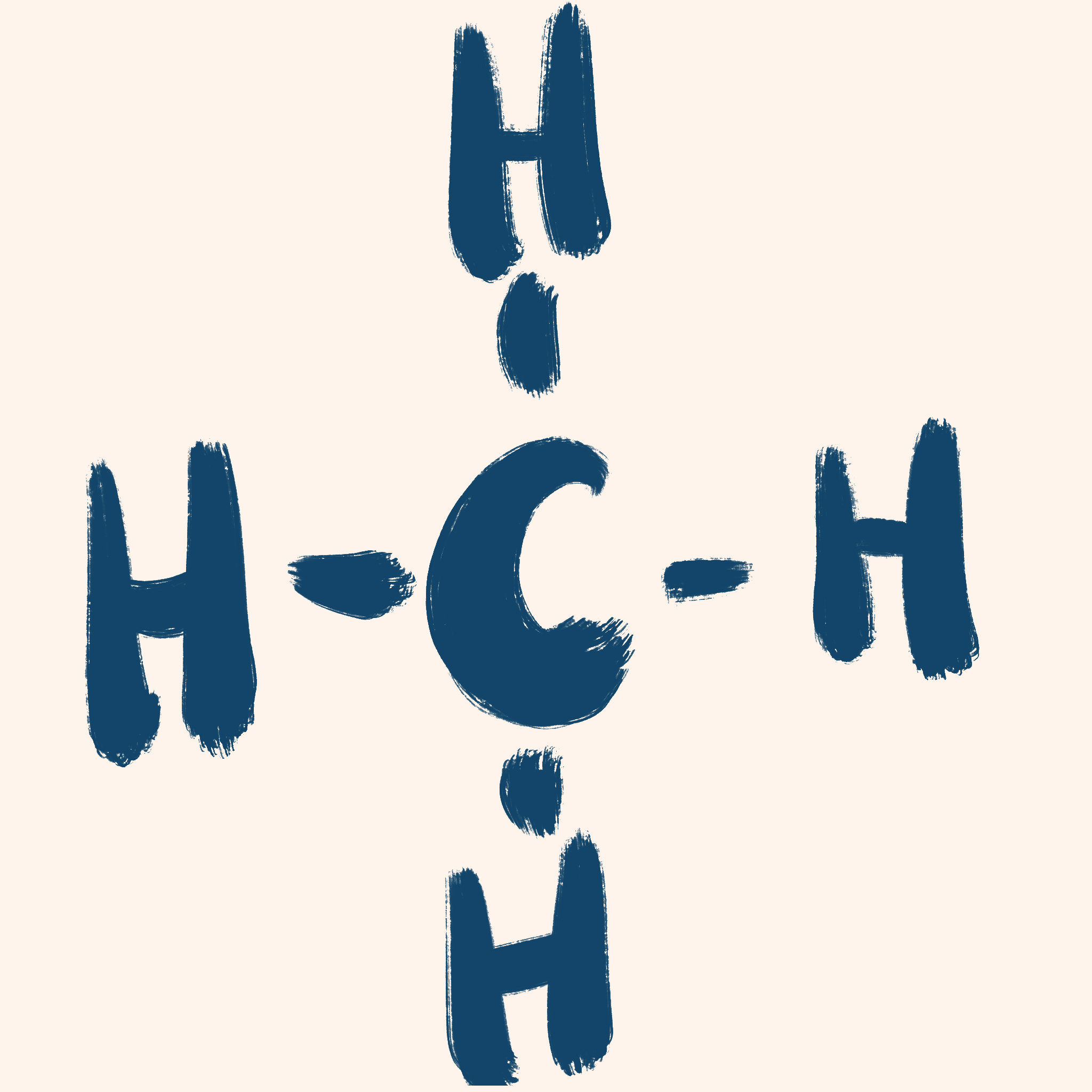
Displayed / structural formula
Important:
H (Hydrogen) has 1 bond
C (Carbon) has 4 bonds
(This will help you draw the structure)
Steps:
- Draw a chain with the correct number of C’s (carbons).
- Add H’s (hydrogens) around the C’s (carbons) to make each carbon have 4 bonds.
- Ensure that the lines are drawn in and that each letter has the correct number of bonds.
Condensed formula
This is similar to the structural formula but it is written.
- Look at the structural formula and write each individual carbon alone.
- Write how many hydrogen atoms are bonded to the individual carbons.
eg. C2H6

- There are 2 C’s
C C - There are 3 H’s bonded to each C
Therefore: CH3CH3
When the “chain of C’s” is longer, there are repeated parts of the formula which you can group together.
eg. C2H12

- There are 5 C’s
C C C C C - There are 3 H’s bonded to the first and last C’s
CH3 C C C CH3 - There are 2 H’s bonded to the other 3 C’s
CH3CH2CH2CH2CH3 - We can group the repeated parts together
Therefore: CH3(CH2)3CH3
Summary

Have any questions about this topic? Leave a comment below and I’ll get back to you 🙂

Leave a Reply